Autoclave validation consists of using chemical and biological indicators to confirm the autoclave is working properly. Chemical indicators are easy to use and typically show if an autoclave was able to reach a minimum temperature but they cannot indicate the length of time the temperature was held. Biological indicators are not as easy to use but can more definitively confirm autoclave sterilizing power as they are both time and temperature dependent.
Chemical Indicators
Chemical indicators for autoclaves are typically designed to visibly change color after exposure to normal autoclave operating temperature (121°C at 15psi). These indicators are only used as a quick reference to show that the autoclave cycle reached the temperature that the indicator was rated for. The typical chemical indicators are sold as small cards, papers, or tape. Indicator cards or paper are usually placed in the center of a load as well as around the periphery of the chamber. Indicator tape is typically used on the outside of all containers that are set to be decontaminated (autoclave bags, autoclave pan, etc). If a chemical indicator does not indicate that the autoclave cycle reached the temperature that the indicator was rated for, the material is not considered decontaminated.

Biological Indicators
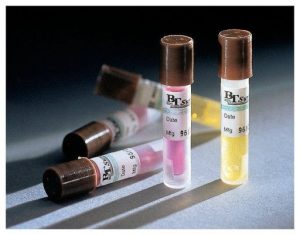
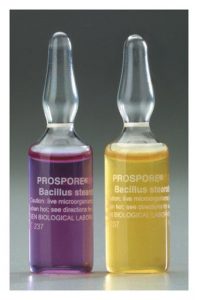
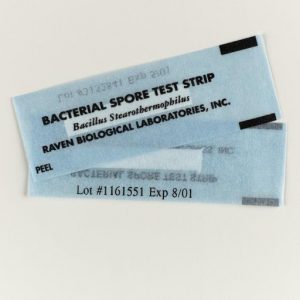
Biological indicators are designed to show that an autoclave cycle was successful at eliminating microorganisms. These indicators utilize bacterial spores such as Geobacillus stearothermophilus which are highly resistant to heat and steam. G. stearothermophilus spores become inactivated when exposed to 121.1°C temperature for more than 20 minutes. This strain can indicate that a cycle was able to reach temperature as well as indicate the minimum length of time the temperature was held. Biological indicators are typically sold as spore strips that must be cultured on media or ampules that do not require culturing. These indicators are normally placed on the bottom shelf closest to the drain as this area is the hardest to sterilize. After the autoclave cycle is complete the biological indicators are required to be incubated according to the manufacturer and compared to a control indicator (which was not included in the cycle). If the test indicator shows signs of microorganism growth such as that found in the control indicator(cloudy media, colony formation) then the validation has failed and the autoclave should be put out of service until it can pass validation. If there is no growth in the control indicator then the validation fails and must be repeated until it can pass validation. If an autoclave fails validation it may require servicing or a change in settings.